|
| |
|
| |
|
|







|
|
TCHS 4O 2000 [4o's nonsense] alvinny [2] - csq - edchong jenming - joseph - law meepok - mingqi - pea pengkian [2] - qwergopot - woof xinghao - zhengyu HCJC 01S60 [understated sixzero] andy - edwin - jack jiaqi - peter - rex serena SAF 21SA khenghui - jiaming - jinrui [2] ritchie - vicknesh - zhenhao Others Lwei [2] - shaowei - website links - Alien Loves Predator BloggerSG Cute Overload! Cyanide and Happiness Daily Bunny Hamleto Hattrick Magic: The Gathering The Onion The Order of the Stick Perry Bible Fellowship PvP Online Soccernet Sluggy Freelance The Students' Sketchpad Talk Rock Talking Cock.com Tom the Dancing Bug Wikipedia Wulffmorgenthaler |
|
bert's blog v1.21 Powered by glolg Programmed with Perl 5.6.1 on Apache/1.3.27 (Red Hat Linux) best viewed at 1024 x 768 resolution on Internet Explorer 6.0+ or Mozilla Firefox 1.5+ entry views: 640 today's page views: 25 (2 mobile) all-time page views: 3394542 most viewed entry: 18739 views most commented entry: 14 comments number of entries: 1227 page created Sun Jul 6, 2025 01:25:41 |
|
- tagcloud - academics [70] art [8] changelog [49] current events [36] cute stuff [12] gaming [11] music [8] outings [16] philosophy [10] poetry [4] programming [15] rants [5] reviews [8] sport [37] travel [19] work [3] miscellaneous [75] |
|
- category tags - academics art changelog current events cute stuff gaming miscellaneous music outings philosophy poetry programming rants reviews sport travel work tags in total: 386 |

| ||
|
Vaccines Continuing, everyone seems to be awaiting salvation through the needle nowadays; "there's no returning to normal life without a working vaccine" is the current received wisdom, with not-unfounded contrarian thought along the lines that - eh, various other major epidemics (e.g. SARS, Zika, Ebola, AIDS) have not been resolved through vaccination, so shouldn't there be more weight on alternative solutions given best-case scenarios for mass vaccinations starting only late next year - largely ignored. Far easier to jump on the bandwagon by taking encouraging news on development where one can, as for the Arcturus vaccine that we've called dibs on. Technically, of course, the world does have multiple working vaccines already - depending on who you're willing to believe. Trial results of a Russian candidate have been published in The Lancet about a month back, with the esteemed medical journal tweeting that it was by all indications "safe and effective". Given that further excitement has been muted, one suspects that many remain wary - The Lancet's staking of their name notwithstanding - and while it might be understood if rival powers are distrusting due to vaccine nationalism, the apparent slow uptake within Russia itself - and the resignation of an involved doctor over ethics - could be instructive. China's Sinovac has declared victory too, with their scientists proclaiming 99% confidence that it would work from Phase 2 trials [N.B. typical Phase 3 failure rates appear over 50%], but it's not like they're waiting for the results, or even asking for permission, anyway. The adventurous might try it out for themselves, but let's just say that circumspection is a virtue. There are obviously risks relating to rushing vaccine trials, with the AstraZeneca adenovirus-based offering already held up due to serious complications on two participants; the Moderna and Pfizer ones have likewise reported heavy side-effects. Historically, being too eager to vaccinate can end in tears, as with Gerald Ford's swine flu vaccine for H1N1 in 1976, or more recently vaccine-derived polio in Africa, and Pandemrix in 2009. That last made over 800 kids brain-damaged, leading to a £60 million settlement in Britain. To The BMJ's credit, they published a letter questioning why the public had not been informed of early trial warnings some nine years on, and it's not like Big Pharma has to worry about that on this occasion, having wangled full waiver of liability in most countries. Trouble is, the line between blind anti-vaxxism and considered prudence can be very fine, and one has to thank The Donald here for returning some forbearance to the fray. By simply tweeting that vaccine development is going a-ok, GEOTUS has singlehandedly turned the establishment stand against "vaccine hesitancy and refusal" on its head, with various media outlets now falling over themselves to inform the public about how even minor side effects are bad, and why vaccines can be dangerous. Whatever it takes to get the word out... Not All Solutions Considered Equal One particularly egregious misconception would have to be that the coronavirus vaccine will be some sort of silver bullet - get vaccinated, and you're immune (like GEOTUS, maybe, probably for awhile at least)! Quite a few seem to have missed the memo that a working vaccine might be only about 50% effective, as warned by Dr. Fauci among other health authorities, with some flu vaccines coming in as low as around 25%... and that masks might actually offer superior protection than vaccines, from the Director of the CDC himself. For what it's worth, the NEJM has just advocated for mandatory vaccination in the face of the majority of the populace being (understandably) reluctant, and it might once more be worthwhile to consider the incentives here. Love him or loathe him, one could be forgiven for figuring that Duterte has a point in accusing Western Big Pharma of being all about the profit and only incidentally about public health, which has resulted in a glaring preference for expensive treatments over cheaper but possibly still-effective ones. As explained with Remdesivir (on which more very soon), a treatment costing thousands of dollars is basically useless to large swathes of the global population, and one can only hope that one of the more cost-effective initiatives works out eventually. The NEJM, by the way, has also involved themselves in the battle to control the narrative at the very highest levels, together with The Lancet, Nature, Science and Scientific American. This struggle was not confined to the ivory-tower journals either, with the FDA Commissioner chastised by the former editors-in-chief of Science and npj Digital Medicine, for misstating that a convalescent plasma treatment would "save 35 lives out of every 100 people who got it" (paraphrased). Turns out, it seems that the poor fellow would have been entirely correct had he just phrased it as "35 out 100 patients who died would have been saved" and emphasized that it was a relative and not an absolute risk reduction - which, it has to be said, appears a routine convention in reporting results (e.g. Gilead press release on Remdesivir (62%!), Science editorial on Dexamethasone (33%!), which all somehow escaped criticism that "...getting to a 35% 'relative' reduction in mortality involves comparing the before and after groups in a way that dramatically exaggerates [?] the effect of the treatment") Personally, one detects a slight hint of desperation here by the legacy set on preserving their monopoly over accepted truth. Perhaps slightly ironically, however, it has oft been regarded as wise to take pronouncements from these very top journals with a pinch of salt, from their especially-high retraction rates. And for the medical Big Four in particular, it almost seems a rite of passage for their former editors-in-chief to pen warnings and books explaining how one really shouldn't believe half of what they publish (I'm working through former NEJM EIC Marcia Angell's The Truth About the Drug Companies) - which might make the ongoing skepticism slightly more palatable. Remdesivir & HCQ Reloaded Which brings us back to the subject of Remdesivir, last covered back in early July. The controversy has quietened considerably since those heady days, with Remdesivir given a boost with an NEJM article reporting that it successfully shortened time to recovery by about five days (10 days vs. 15 days for placebo), together with a statistically significant reduction in mortality, on a total of about 1,000 patients. And all was well and good, until the WHO's SOLIDARITY trial dropped its bombshell - Remdesivir had not been found to have any significant effect on either recovery time or mortality, from a trial arm involving 2,750 patients. Gilead obviously wasn't pleased about this declaration, but hey, they've made their sales; there's word that they received early notice of the SOLIDARITY trial's negative result in September, but went ahead to sign a billion-dollar deal with the European Union on October 8 anyhow (that's now rightly facing calls for review, after this latest revelation). Perhaps they might consider getting IMMUNE GEOTUS to vouch for it? Let's talk a little methodology here. Scientists can investigate much the same phenomena, and return with divergent results (and conclusions); oftentimes, this is correct, and only to be expected. Especially in social and biological fields, there are just too many unknowns and uncontrollable variables, for even competent and well-intentioned investigators to be guaranteed to concur, without any wrongdoing on anybody's end. 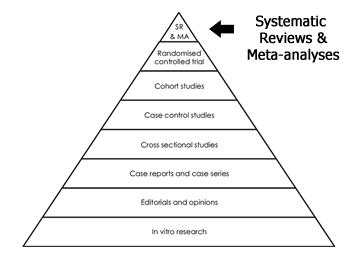 Hierarchy of Evidence (Source: researchgate.net) Therefore, accepted practice is to defer to a hierarchy of evidence in appraising a claim. It begins near the bottom with editorials and opinions (which might be influential, but at the end, everyone has one, and patients should seek a second one if they so desire), rises through case reports (i.e. N=1, oft by bright-eyed residents seeking to get their feet wet), then (retrospective) cross-sectional studies, case-control studies, cohort studies and then the much-ballyhooed randomized controlled trial (RCT). Each of these study designs has their uses, and one suspects that some accommodations might be made for study parameters (e.g. is an RCT of 100 patients definitely better evidence than a cross-sectional study on a million patients?), but that covers the gist of it. The capstone of the pyramid is given as systematic reviews and meta-analyses, which makes sense since multiple RCTs (and other studies) provide more information than a single RCT, assuming they are all well-conducted (i.e. the Gilead-conducted RCT published in NEJM versus the SOLIDARITY trial and others, as described above). Consider a particularly delicate experiment on the efficacy of a drug, that's been assigned to a hundred investigators; perhaps 80 of them return with a positive effect, and 20 with no effect, after their best efforts. One might consider this a promising avenue, looking at the whole picture (there may be a bias against negative results in published literature, but that's another story) Still on the proposed scenario, note that it remains easy for an advocate against the drug to argue that it doesn't work - simply pick out the 20 negative experiments as (entirely valid) evidence, while ignoring the other 80 (of course, it works the other way too, but in this case the proponents would probably not have to resort to that). Thus, the importance of being comprehensive in identifying relevent work in a systematic review, which usually involves carefully defining the search terms and databases/archives used in procuring publications.  HCQ Outpatient RCT outcomes (Source: threadreaderapp.com) With all this background in mind, allow me to cover some such systematic reviews on HCQ, that have emerged in the few months since it last made waves around July. One particularly highly-circulated example (at least on Reddit) was Fiolet et al.'s "Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis" from Clinical Microbiology and Infection in August, which was promoted as bolstering the stand that HCQ was not associated with reduced mortality in hospitalized patients (as indeed was its conclusion). Overall, it indeed seems a fine example of a well-constructed systematic review (note thorough description of the study selection process, which winnowed an initial 839 papers, down to just 27 that were used to assess the use of HCQ alone) Figure 2 summarizes the main analysis - including individual risk ratios (RR) and weights for each included paper - and produces a combined random effects model risk ratio of 0.83 [95% C.I. 0.65-1.06]. Now, one believes that a risk ratio of 0.83 means that those who underwent the treatment (HCQ) had 0.83 times the risk of COVID-19 mortality, compared to the controls... which is, like, good? However, it is also true that this apparent improvement was not significant, due to the 95% C.I. containing 1.00 (it is further mentioned in the paper that "...after inclusion of studies with critical risk of bias, the global RR was marginally not significant 0.80 [95% CI 0.65-1.00]") Looking elsewhere, there exist various preprints of other systematic reviews on HCQ, with one by Harvey Risch and associates finding a 24% reduction in COVID-19 infection, hospitalization or death with HCQ, Garcia-Albeniz et al. coming up with a pooled risk ratio estimate of 0.78 [95% C.I. 0.61-0.99] (just about significant, and not that far away from Fiolet et al.), and another by Prodromos & Rumschlag concluding "consistent clinical efficacy for COVID-19 when it is used early in the outpatient setting", and that HCQ would "in general... appear to work better the earlier it is used". There's also an interesting living aggregation available hosted at c19study.com, which touts a 100% positive (or more accurately, non-negative) effect of HCQ in early treatment, with a 64% median improvement, and a 26% median improvement with late treatment as of currently (which seems broadly in line with the systematic reviews above). Now, there are some legitimate criticisms, such as the webpage possibly excluding studies and not weighing them by strength of evidence, though other complaints such as it listing preprints might be expecting too much (Fiolet et al. includes preprints too, for example, and given typical journal publishing turnaround times, one could expect some otherwise entirely good papers to be held up), but at some point one thinks it would be reasonable for the critics to substantiate their accusations - where are all the countervailing studies showing that early HCQ treatment, at conventional dosages, is harmful? From all the vitriol the medication has attracted, surely it wouldn't be hard to list them out? Talking Past One Another Rewinding back to late July, a group of physicians calling themselves America's Frontline Doctors held an open interview outside Capitol Hill, claiming that HCQ (and zinc, and Zithromax) was a "cure" for the coronavirus; now, while "cure" is a loaded word that can be taken to imply 100% effectiveness, it is hardly outside the realm of plausibility that HCQ might well help - all the more if prescribed early - from the reviews described above. These practising doctors would be swiftly suspended from major social media outlets for their pains - which extended to prominent persons who simply retweeted their video - and whatever one's personal stance is about the HCQ issue, the implications for free speech are chilling. Twitter, for example, referred to their COVID-19 misinformation policy in their censorship, but this only begs the question of what authority they are deferring to, in determining what's misinformation and what's not. If it's the WHO or the CDC or similar, then it could easily be pointed out that these organizations have routinely - if unintentionally - doled out misleading advice themselves. One of the group, for instance, was from the Henry Ford Health System that had published a study on 2,541 patients suggesting a substantial reduction in mortality with standard HCQ dosing, and if a fellow isn't allowed to advocate for one's own findings to begin with - right or wrong as he might later turn out to be - how can viewpoints outside the establishment line (which can be based on very shaky foundations, as seen with #Lancetgate), ever gain a hearing? We'll come to the consequences of censorship on social media again, and turn attention to the derision of two doctors opposed to AFD's HCQ advocacy; one rebuttal is particularly interesting: "I'm an ER doc that treated #COVID in NYC & AZ. We gave #hydroxychloroquine and it didn't work. Same with Azithromycin. Does this 'Dr' even treat CRITICAL hypoxic patients? Or just healthy patients walking in her office. Disgusting!" One might easily agree with the emergency room docs - on dying, late-stage patients, next to nothing has been proven to work reliably, from the latest SOLIDARITY data and other studies. However, the jibe at treating only "healthy patients" strikes one as slightly strange. In general, isn't the idea to treat a virus as early as possible, rather than wait until the patient is fighting for his life? Indeed, the early advocates for HCQ have consistently been encouraging early treatment, since the idea was to slow or stop viral replication before the patient's organs had been overwhelmed, after which application of HCQ and other medications were far more likely to be detrimental. Given this, perhaps both sides have a tenable point? This fine if rather simple distinction appears to have been mostly lost amongst the medical and scientific communities' shining stars, alas, with Science Translational Medicine's ongoing dismissiveness of HCQ from the SOLIDARITY trial's findings pointing to a meta-analysis... comprised of 16 unpublished trials (not including preprints), and 10 publications/preprints - but again, this could be seen as a bit of misdirection to begin with, given that SOLIDARITY remains a test of late-stage efficacy with high dosages. The entire affair is exceedingly odd in retrospect:
Next: Apotusgetics |
||||||
 Copyright © 2006-2025 GLYS. All Rights Reserved. |
||||||